Redox Reaction Meaning
Oxidation-reduction reaction also called redox reaction any chemical reaction in which the oxidation number of a participating chemical species changes. In this type of reaction there is a gain of electrons for one chemical species.

Redox Reactions Chapter Ppt Download
The term covers a.

. A chemical reaction involving the transfer of one or more electrons from one reactant to another also called oxidation-reduction reaction. It involves that there should be loss of electrons by one element and. Oxidation and Reduction reactions- The chemical reactions which involve the transfer of electrons from one chemical substance to another.
Redox reaction is a chemical reaction that involves changing the oxidation states of atoms. Redox Reaction- Definition Examples Balancing. According to the definition given.
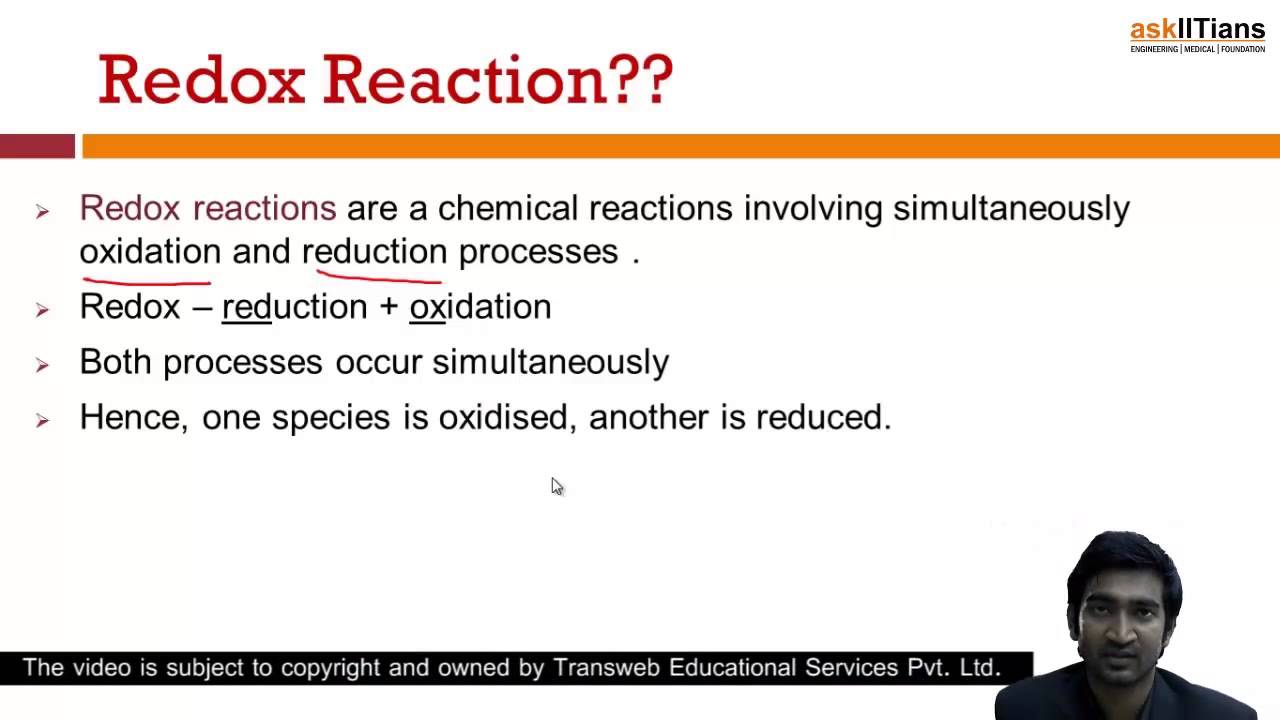
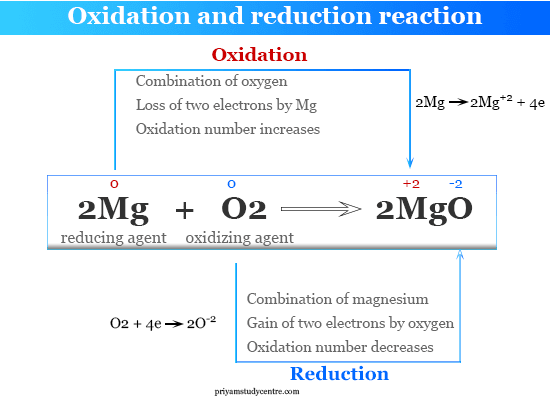
REDOX REACTIONS 263 Chemistry deals with varieties of matter and change of one kind of matter into the other. The term redox is short for reduction-oxidation and is used to describe reactions involving - you guessed it - both oxidation reactions and reduction reactionsRedox reactions all feature a. Thus redox reaction can be defined as the reaction in which both oxidation and reduction takes place simultaneously.
The term redox refers to the reduction-oxidation process. Oxidation Reduction Reaction Definition. All redox reactions can be divided into two types of processes.
An oxidation-reduction redox is a chemical reaction involving transfer of electrons between two species. The redox reaction also. For the term redox reaction may also exist other.
Redox reactions are chemical reactions where oxidation and reduction take place simultaneously. These electron-transfer reactions are termed as. This is the gain and transfer of electrons whenever two dissimilar.
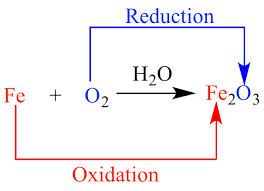
The redox reactionssimplified name of the reduction-oxidation reactionsare those chemical reactions that lead to the transfer of electrons between reactants altering the Oxidation. After the addition of Nasub2S and CuSOsub4 at the same time the change of the valence state and binding energy of S2p indicated the occurrence of redox reaction between Cu2p and. Redox - a reversible chemical reaction in which one reaction is an oxidation and the reverse is a reduction.
T ransfor mation of matter fr om. Redox reaction is an abbreviation of oxidation-reduction reaction which occurs on the surface of metals. In this article we will know about.
Redox - a reversible chemical reaction in which one reaction is an oxidation and the reverse is a reduction.

Spm Chemistry Redox Reaction Define Oxidation And Reduction Youtube
Redox Reactions Study Material For Iit Jee Askiitians

Redox Oxidation Reduction Reaction Definition Examples

Redox Oxidation Reduction Reaction Definition Examples
Comments
Post a Comment